The non motor symptoms of PD are so important. This study shows how autonomic dysfunction worsens significantly over short periods of follow up in patients that have a diagnosis of PD. We already know from other studies that autonomic dysfunction is a marker of Parkinson's disease severity and heralds a shorter survival of patients with PD.
There has been quite a lot of focus on sleep, cognition and mood as non motor features of PD but we are not adequately addressing symptoms and consequences of autonomic dysfunction and studies such as this highlight the importance. Previously, marked autonomic involvement used to raise the suspicion of an alternative parkinsonian disease (such as multiple systems atrophy) and although autonomic dysfunction is a prominent feature of MSA, it is also very prevalent in PD.
Of course autonomic dysfunction also often precedes a diagnosis of PD, whether in the form of orthostatic hypotension, or more commonly with constipation, erectile dysfunction or urinary symptoms. We at PREDICT-PD have a longstanding interest in how symptoms of autonomic dysfunction can be used to identify those that might one day go on to be diagnosed with PD...
Alastair Noyce
Mov Disord. 2017 Dec 26. doi: 10.1002/mds.27268. [Epub ahead of print]
Merola A1, Romagnolo A2, Rosso M1, Suri R1, Berndt Z1, Maule S3, Lopiano L2, Espay AJ1.
http://onlinelibrary.wiley.com/doi/10.1002/mds.27268/full
BACKGROUND:
Dysautonomia is a frequent and disabling complication of PD, with an estimated prevalence of 30-40% and a significant impact on the quality of life.
OBJECTIVES:
To evaluate the rate of progression of dysautonomia and, in particular, orthostatic hypotension, in a cohort of unselected PD patients, and assess the extent to which the progression of dysautonomia affects activities of daily living, health-related quality of life, and health care utilization in PD.
METHODS:
We recruited 131 consecutive patients into a 12-month, prospective, observational cohort study. Clinical measures included the International Parkinson and Movement Disorder Society/UPDRS, the Scale for Outcomes in Parkinson Disease-Autonomic, the Orthostatic Hypotension Symptoms Assessment, and orthostatic blood pressure measurements. Health care utilization was quantified as the number of hospitalizations, emergency room visits, and outpatient clinic evaluations.
RESULTS:
The overall severity of autonomic symptoms, as measured by the the Orthostatic Hypotension Symptoms Assessment total score, worsened by 20% over 12 months (P < 0.001), with an overall increase in orthostatic hypotension prevalence from 31.1% to 46.7% (P < 0.001). Worsening of autonomic symptoms was independently associated with deterioration in daily living activities (P = 0.021) and health-related quality of life (P = 0.025) adjusting for disease duration, cognitive impairment, and motor severity. Regardless of symptomatic status, orthostatic hypotension was associated with greater deterioration in daily living activities, health care utilization, and falls (P ≤ 0.009) compared to patients without orthostatic hypotension.
CONCLUSIONS:
The severity of autonomic symptoms progressed by 20% over 1 year and was independently associated with impairments in daily living activities and health-related quality of life. Symptomatic and asymptomatic orthostatic hypotension were both associated with increased prevalence of falls and health care utilisation.
© 2017 International Parkinson and Movement Disorder Society.
Welcome to the blog for the PREDICT-PD project. We are working to understand the risk factors for Parkinson's Disease and blogging about advances made in prediction and early detection of the disease.
Thursday 28 December 2017
Friday 15 December 2017
Update on REM sleep behaviour disorder
This is a great review of the latest understanding about REM sleep behaviour disorder (RBD), which has been closely associated with risk for Parkinson's disease. The condition was first described in cats with damage to the midbrain, the area in the brainstem above the substantia nigra, where the cells damaged in Parkinson's disease reside. The hallmark of the condition is loss of normal muscle paralysis during REM sleep, the state in which most dreams occur, so that dreams are acted out.
In itself the condition can be more or less benign, with some patients hardly noticing any issues and others harming themselves badly at night. But importantly, it is now clearly linked with Parkinson's disease - many patients with Parkinson's go on to get the disorder and patients with RBD are at higher risk of going on to get Parkinson's. Increasing evidence shows a common underlying disease process, with build up of the protein alpha-synuclein. As a risk factor, unlike age or smell loss, it is highly specific for Parkinson's, or the related condition lewy body dementia. In patients with RBD, many have similarities to people with Parkinson's, without yet having developed movement problems - for example, there are imaging changes, autonomic changes and cognitive changes.
In the search for disease-modifying treatment, this group is likely to be very valuable - with the high rate of conversion to Parkinson's, the benefits of treatment are more likely to outweigh risks than for the general population. Continuing to characterise these patients and find the measures most sensitive to progression will ultimately enable us to recruit for clinical trials for preventative medications.
Nat Rev Neurol. 2017 Nov 24. doi: 10.1038/nrneurol.2017.157. [Epub ahead of print]
Idiopathic REM sleep behaviour disorder and neurodegeneration - an update.
Högl B, Stefani A, Videnovic A
Abstract
Key points
- Clinically isolated rapid eye movement (REM) sleep behavioural disorder (RBD) is considered to be an early stage of α-synucleinopathy that can provide a window into the future health of the brain
- Elementary, minor and major body and limb jerks on surface electromyography (REM sleep without atonia) or video polysomnography are the main hallmarks of RBD
- Accurate diagnosis of isolated RBD is critical in clinical trials and should be confirmed by polysomnography rather than implied by subjective tools such as questionnaires
- In patients with isolated RBD, conversion to α-synucleinopathy often results in Parkinson disease dementia or dementia with Lewy bodies; the highest risk of conversion has been calculated for polysomnography-confirmed isolated RBD
- The term 'prodromal RBD' should be used (in analogy to prodromal Parkinson disease) to define recognizable precursory disease states before the full diagnostic criteria for isolated RBD are met
- This prodromal stage might enable the identification of individuals at risk of neurodegeneration even before the development of isolated RBD
Wednesday 13 December 2017
Detecting anxiety in individuals with Parkinson disease: A systematic review
This is a new systematic review of studies assessing the diagnostic accuracy of anxiety scales in people with PD. It is important because anxiety is a common feature of Parkinson's both before and after the diagnosis. The authors found that overall 30% of people with Parkinson's meet the criteria for anxiety, but I have to say that in my experience I would put it closer to 50%. Maybe this reflects a bias in the patients I see??
The properties of the scales available for assessing anxiety are important. At the beginning of PREDICT-PD, we went through a pain-staking process of selection and in the end arrived at the Hospital Anxiety Depression Scale (HADS). In fact, at that time we knew that it was not the very best Anxiety scale or the very best Depression scale in terms of diagnostic accuracy, but it did combine assessment of both problems in one validated questionnaire, it was feasible to self-administer and it was relatively short. Nearly seven years on I am still happy with that decision but we may now test other scales within the PREDICT-PD programme too. Depression and anxiety are two features that are likely to pre-date a diagnosis of PD by a long time and it is critically important to be using the best tools to identify them both. They also have a dramatic impact on quality of life and are treatable, which are two good reasons to be trying to pick them up in people that don't have a diagnosis because there are likely to be significant health benefits...
Alastair Noyce
Neurology. 2017 Dec 6. pii: 10.1212/WNL.0000000000004771. doi: 10.1212/WNL.0000000000004771. [Epub ahead of print]
Mele B, Holroyd-Leduc J, Smith EE, Pringsheim T, Ismail Z, Goodarzi Z.
http://n.neurology.org/content/early/2017/12/06/WNL.0000000000004771.long
OBJECTIVE: To examine diagnostic accuracy of anxiety detection tools compared with a gold standard in outpatient settings among adults with Parkinson disease (PD).
METHODS: A systematic review was conducted. MEDLINE, EMABASE, PsycINFO, and Cochrane Database of Systematic Reviews were searched to April 7, 2017. Prevalence of anxiety and diagnostic accuracy measures including sensitivity, specificity, and likelihood ratios were gathered. Pooled prevalence of anxiety was calculated using Mantel-Haenszel-weighted DerSimonian and Laird models.
RESULTS: A total of 6,300 citations were reviewed with 6 full-text articles included for synthesis. Tools included within this study were the Beck Anxiety Inventory, Geriatric Anxiety Inventory (GAI), Hamilton Anxiety Rating Scale, Hospital Anxiety and Depression Scale-Anxiety, Parkinson's Anxiety Scale (PAS), and Mini-Social Phobia Inventory. Anxiety diagnoses made included generalized anxiety disorder, social phobia, and any anxiety type. Pooled prevalence of anxiety was 30.1% (95% confidence interval 26.1%-34.0%). The GAI had the best-reported sensitivity of 0.86 and specificity of 0.88. The observer-rated PAS had a sensitivity of 0.71 and the highest specificity of 0.91.
CONCLUSIONS: While there are 6 tools validated for anxiety screening in PD populations, most tools are only validated in single studies. The GAI is brief and easy to use, with a good balance of sensitivity and specificity. The PAS was specifically developed for PD, is brief, and has self-/observer-rated scales, but with lower sensitivity. Health care practitioners involved in PD care need to be aware of available validated tools and choose one that fits their practice.
The properties of the scales available for assessing anxiety are important. At the beginning of PREDICT-PD, we went through a pain-staking process of selection and in the end arrived at the Hospital Anxiety Depression Scale (HADS). In fact, at that time we knew that it was not the very best Anxiety scale or the very best Depression scale in terms of diagnostic accuracy, but it did combine assessment of both problems in one validated questionnaire, it was feasible to self-administer and it was relatively short. Nearly seven years on I am still happy with that decision but we may now test other scales within the PREDICT-PD programme too. Depression and anxiety are two features that are likely to pre-date a diagnosis of PD by a long time and it is critically important to be using the best tools to identify them both. They also have a dramatic impact on quality of life and are treatable, which are two good reasons to be trying to pick them up in people that don't have a diagnosis because there are likely to be significant health benefits...
Alastair Noyce
Neurology. 2017 Dec 6. pii: 10.1212/WNL.0000000000004771. doi: 10.1212/WNL.0000000000004771. [Epub ahead of print]
Mele B, Holroyd-Leduc J, Smith EE, Pringsheim T, Ismail Z, Goodarzi Z.
http://n.neurology.org/content/early/2017/12/06/WNL.0000000000004771.long
OBJECTIVE: To examine diagnostic accuracy of anxiety detection tools compared with a gold standard in outpatient settings among adults with Parkinson disease (PD).
METHODS: A systematic review was conducted. MEDLINE, EMABASE, PsycINFO, and Cochrane Database of Systematic Reviews were searched to April 7, 2017. Prevalence of anxiety and diagnostic accuracy measures including sensitivity, specificity, and likelihood ratios were gathered. Pooled prevalence of anxiety was calculated using Mantel-Haenszel-weighted DerSimonian and Laird models.
RESULTS: A total of 6,300 citations were reviewed with 6 full-text articles included for synthesis. Tools included within this study were the Beck Anxiety Inventory, Geriatric Anxiety Inventory (GAI), Hamilton Anxiety Rating Scale, Hospital Anxiety and Depression Scale-Anxiety, Parkinson's Anxiety Scale (PAS), and Mini-Social Phobia Inventory. Anxiety diagnoses made included generalized anxiety disorder, social phobia, and any anxiety type. Pooled prevalence of anxiety was 30.1% (95% confidence interval 26.1%-34.0%). The GAI had the best-reported sensitivity of 0.86 and specificity of 0.88. The observer-rated PAS had a sensitivity of 0.71 and the highest specificity of 0.91.
CONCLUSIONS: While there are 6 tools validated for anxiety screening in PD populations, most tools are only validated in single studies. The GAI is brief and easy to use, with a good balance of sensitivity and specificity. The PAS was specifically developed for PD, is brief, and has self-/observer-rated scales, but with lower sensitivity. Health care practitioners involved in PD care need to be aware of available validated tools and choose one that fits their practice.
Tuesday 12 December 2017
What can we learn from Huntington's Disease therapy?
It's great to see our UCL colleagues in the news this week with a potential breakthrough in neurodegenerative disease - for the first time a therapy has been shown to lower levels of the damaging huntingtin protein in Huntington's disease
Of course, the headlines mention potential to see this therapy used in other neurodegenerative disease. But could a similar 'gene silencing' therapy have any use in Parkinson's? The timely review below summarises the current situation with antisense oligonucleotide (ASO) therapy. These drugs target RNA, the product of DNA. Usually RNA goes on to produce proteins but by binding to specific forms of RNA, protein production can be prevented or altered. Development of these molecules has led to highly specific and minimally toxic agents that seem to be ideally suited to address the protein build-up found in neurodegenerative disease. ASOs have shown very positive results in the childhood condition Spinal Muscular Atrophy, where Nusinersen reduced risk of death or invasive ventilation by 47%.
One of the main challenges in neurological disease is that these molecules can't cross between the blood and the brain, meaning they currently have to be delivered directly into the spinal column. Another challenge for Parkinson's is that most cases of the disease are not caused by a specific gene defect - in rare cases with alpha-synculein mutations, ASOs aiming to silence the gene by switching off protein production may be useful but this is unlikely to be useful for most patients.
However, the potential of these molecules to interact with proteins in multiple ways - either halting protein production, changing the type of protein produced or restoring healthy protein production has huge potential. We eagerly await the follow up results from the Huntington's study and will continue to follow this story closely.
Nat Rev Neurol. 2017 Dec 1. doi: 10.1038/nrneurol.2017.148. [Epub ahead of print]
Antisense oligonucleotides: the next frontier for treatment of neurological disorders.
Rinaldi C Wood MJA
Monday 11 December 2017
The BRAIN test: a keyboard-tapping test to assess disability and clinical features of multiple sclerosis
Okay. Not strictly Parkinson's research but the BRAIN tap test comes from the PREDICT-PD team. Here we show that the BRAIN test can be used for detecting symptoms of Multiple Sclerosis (another disease of the nervous system).
This gives support to the notion that the BRAIN test may be able to differentiate people with subtle signs of Parkinson's as opposed to those that have MS or stroke... the characteristics of alternate finger tapping may be different between these groups of patients.
The BRAIN test is already being used in a number of observational studies and a couple of clinical trials....
J Neurol. 2017 Dec 4. doi: 10.1007/s00415-017-8690-x. [Epub ahead of print]
Shribman S1, Hasan H2, Hadavi S3, Giovannoni G4, Noyce AJ5,6. Author information Abstract
https://link.springer.com/article/10.1007%2Fs00415-017-8690-x
BACKGROUND: The BRadykinesia Akinesia INcordination (BRAIN) test is an online keyboard-tapping test previously validated as a sensitive tool for detecting signs of Parkinson's disease.
OBJECTIVES: To determine whether the BRAIN test can measure disability in MS and identify the presence of pyramidal or cerebellar dysfunction.
METHODS: Kinesia scores (KS, number of key taps in 30 s), akinesia times (AT, mean dwell time on each key) and incoordination scores (IS, variance of travelling time between keys) were calculated in 39 MS patients. These were correlated against the Expanded Disability Status Scale (EDSS) scores, pyramidal and cerebellar functional system scores and 9-hole peg test scores.
RESULTS: EDSS correlated with KS (r = - 0.594, p < 0.001), AT (r = 0.464, p = 0.003) and IS (r = 0.423, p = 0.007). 9-HPT scores strongly correlated with KS (r = 0.926, p < 0.001). Pyramidal scores correlated with KS (r = - 0.517, p < 0.001). Cerebellar scores correlated with KS (r = - 0.665, p < 0.001), AT (r = 0.567, p < 0.001) and IS (r = 0.546, p = 0.007). Receiver operating characteristic curves demonstrate that KS can distinguish between the presence or absence of pyramidal and cerebellar dysfunction with area under curve 0.840 (p < 0.001) and 0.829 (p < 0.001), respectively.
CONCLUSIONS: The BRAIN test can remotely measure disability in MS. Specific scores differ according to the presence and severity of pyramidal or extrapyramidal dysfunction. It demonstrates huge potential in monitoring disease progression in clinical trials.
This gives support to the notion that the BRAIN test may be able to differentiate people with subtle signs of Parkinson's as opposed to those that have MS or stroke... the characteristics of alternate finger tapping may be different between these groups of patients.
The BRAIN test is already being used in a number of observational studies and a couple of clinical trials....
J Neurol. 2017 Dec 4. doi: 10.1007/s00415-017-8690-x. [Epub ahead of print]
Shribman S1, Hasan H2, Hadavi S3, Giovannoni G4, Noyce AJ5,6. Author information Abstract
https://link.springer.com/article/10.1007%2Fs00415-017-8690-x
BACKGROUND: The BRadykinesia Akinesia INcordination (BRAIN) test is an online keyboard-tapping test previously validated as a sensitive tool for detecting signs of Parkinson's disease.
OBJECTIVES: To determine whether the BRAIN test can measure disability in MS and identify the presence of pyramidal or cerebellar dysfunction.
METHODS: Kinesia scores (KS, number of key taps in 30 s), akinesia times (AT, mean dwell time on each key) and incoordination scores (IS, variance of travelling time between keys) were calculated in 39 MS patients. These were correlated against the Expanded Disability Status Scale (EDSS) scores, pyramidal and cerebellar functional system scores and 9-hole peg test scores.
RESULTS: EDSS correlated with KS (r = - 0.594, p < 0.001), AT (r = 0.464, p = 0.003) and IS (r = 0.423, p = 0.007). 9-HPT scores strongly correlated with KS (r = 0.926, p < 0.001). Pyramidal scores correlated with KS (r = - 0.517, p < 0.001). Cerebellar scores correlated with KS (r = - 0.665, p < 0.001), AT (r = 0.567, p < 0.001) and IS (r = 0.546, p = 0.007). Receiver operating characteristic curves demonstrate that KS can distinguish between the presence or absence of pyramidal and cerebellar dysfunction with area under curve 0.840 (p < 0.001) and 0.829 (p < 0.001), respectively.
CONCLUSIONS: The BRAIN test can remotely measure disability in MS. Specific scores differ according to the presence and severity of pyramidal or extrapyramidal dysfunction. It demonstrates huge potential in monitoring disease progression in clinical trials.
Thursday 7 December 2017
Out of the mouths of babes and sucklings
There is an ongoing debate about where Parkinson’s starts. The most
accepted hypothesis of Braak and colleagues suggests that it starts outside the
brian, either in the olfactory (smelling) nerve, or potentially in the gut. Some evidence suggested that people who had had surgery to cut the nerve to
their stomach to treat stomach ulcers were at lower risk of Parkinson’s, which added weight to the theory that Parkinson’s
might ‘ascend’ through the nerves of the gut to the brain. This fits nicely with the
theory that changes in the microbiome (the overall ecosystem of bacteria and
other microorganisms) in the gut plays a part in Parkinson’s. However, this
finding has been disputed by other studies.
A report published this month, continues the story elsewhere in the digestive tract. Using the highly reliable
public health records of the Danish health and civil registration scheme, they tried
to find a relationship between having a tonsillectomy and future risk of
Parkinson’s. They examined ther records of over a million people, including
195,000 who’d had tonsillectomy, primarily in childhood. They found 100 people
who developed Parkinson’s from the tonsillectomy group at a rate of 0.31 (true
figure in the region of 0.22-0.34) cases of Parkinson’s per 100,000 person-years,
and 568 cases of Parkinson’s in the comparison group at 0.27 (true figure in
the region of 0.29-0.34) cases of Parkinson’s per 100,000 person-years. There
was no significant difference between the two groups. Therefore, there is no
evidence of any effect of tonsillectomy on the risk of developing Parkinson’s.
This is an important negative finding, and the editor of Movement
Disorders should be congratulated on publishing it. It is notoriously difficult
to publish ‘negative’ studies, as they rarely make headlines in the broadsheets
(when was the last time you read that X had no effect on Y, compared to the
last story you read claiming that your favourite food put you at risk of …)
Although they found no evidence of an effect, that still isn’t quite the same
of finding evidence of no effect; and so the debate rages on.
RNR
Tonsillectomy and Risk of Parkinson’s Disease: A Danish
Nationwide Population-Based Study
Svensson
E1,2, Henderson VW1,3,4, Szépligeti S1, Stokholm MG5, Klug TE6, Sørensen HT1,3, Borghammer P5.
ABSTRACT
Background: We hypothesized that tonsillectomy
modifies the risk of PD.
Objectives: To test the hypothesis in a
nationwide population-based cohort study.
Methods: We used Danish medical registries to
construct a cohort of all patients in Denmark with an operation code of
tonsillectomy 1980-2010 (n = 195,169) and a matched age and sex general
population comparison cohort (n = 975,845). Patients were followed until PD
diagnosis, death, censoring, or end of follow-up 30 November 2013. Using Cox
regression, we computed hazard ratios for PD and corresponding 95% confidence
intervals, adjusting for age and sex by study design, and potential confounders.
Results: We identified 100 and 568 patients
diagnosed with PD among the tonsillectomy and general population comparison
cohort, respectively, finding similar risks of PD (adjusted hazard ratio = 0.95
[95% confidence interval: 0.76-1.19]; for > 20 years' follow-up (adjusted
hazard ratio = 0.96 [95% confidence interval: 0.64-1.41]).
Conclusion: Tonsillectomy is not associated with
risk of PD, especially early-onset PD. © 2017 International Parkinson and
Movement Disorder Society
Monday 4 December 2017
Patient and Public Involvement
Here at PREDICT-PD we are very keen to ensure we are not just answering abstract questions, but are focussing on the needs on the Parkinson's community. It was a real pleasure to meet with an engaged and dynamic group of people with Parkinson's and a representative of the Cure Parkinson's Trust.
Together, we will ensure that our research is always focussing on what is important: how can we beat Parkinson's? We shared our frustrations that despite the effort that is spent on medical research, we still have so far to go to realise our goal. The scientific method is one of small steps and incremental advances.
Hand-in-hand with this process of understanding the disease in order to create new treatments, are 'fast-track' processes, such as the Linked Trials Initiative, which the Cure Parkinson's Trust is involved with. The Linked Clinical Trials initiative has reviewed the literature to identify 10 drugs that are already licensed for other conditions, such as statins for heart health, exenatide for diabetes and others, and assessed their likelihood to be effective in the fight against Parkinson's, based on sound biological plausibility and early experimental data.
This initiative fits wonderfully with the aims of PREDICT-PD. These compounds may be shown to be effective in established Parkinson's, but just as blowing out a candle is easier than putting out a forest fire, so too changing the course of Parkinson's is likely to be easier and potentially more effective if it is done before the onset of the motor symptoms of the condition.
So, I personally look forward to meeting this group again, being challenged by their insightful questions, being inspired by their experiences and being motivated by their desire for answers!
RNR
ncbi.nlm.nih.gov/pmc/articles/PMC4318242/
Linked Clinical Trials – The Development of New Clinical Learning Studies in Parkinson’s Disease Using Screening of Multiple Prospective New Treatments
Finding new therapies for Parkinson’s disease (PD) is a slow process. We assembled an international committee of experts to examine drugs potentially suitable for repurposing to modify PD progression. This committee evaluated multiple drugs currently used, or being developed, in other therapeutic areas, as well as considering several natural, non-pharmaceutical compounds. The committee prioritized which of these putative treatments were most suited to move immediately into pilot clinical trials. Aspects considered included known modes of action, safety, blood-brain-barrier penetration, preclinical data in animal models of PD and the possibility to monitor target engagement in the brain. Of the 26 potential interventions, 10 were considered worth moving forward into small, parallel ‘learning’ clinical trials in PD patients. These trials could be funded in a multitude of ways through support from industry, research grants and directed philanthropic donations. The committee-based approach to select the candidate compounds might help rapidly identify new potential PD treatment strategies for use in clinical trials.
Together, we will ensure that our research is always focussing on what is important: how can we beat Parkinson's? We shared our frustrations that despite the effort that is spent on medical research, we still have so far to go to realise our goal. The scientific method is one of small steps and incremental advances.
Hand-in-hand with this process of understanding the disease in order to create new treatments, are 'fast-track' processes, such as the Linked Trials Initiative, which the Cure Parkinson's Trust is involved with. The Linked Clinical Trials initiative has reviewed the literature to identify 10 drugs that are already licensed for other conditions, such as statins for heart health, exenatide for diabetes and others, and assessed their likelihood to be effective in the fight against Parkinson's, based on sound biological plausibility and early experimental data.
This initiative fits wonderfully with the aims of PREDICT-PD. These compounds may be shown to be effective in established Parkinson's, but just as blowing out a candle is easier than putting out a forest fire, so too changing the course of Parkinson's is likely to be easier and potentially more effective if it is done before the onset of the motor symptoms of the condition.
So, I personally look forward to meeting this group again, being challenged by their insightful questions, being inspired by their experiences and being motivated by their desire for answers!
RNR
ncbi.nlm.nih.gov/pmc/articles/PMC4318242/
Linked Clinical Trials – The Development of New Clinical Learning Studies in Parkinson’s Disease Using Screening of Multiple Prospective New Treatments
Brundin P, Barker RA, Conn PJ, Dawson TM, Kieburtz K, Lees AJ, et al. Linked Clinical Trials – The Development of New Clinical Learning Studies in Parkinson’s Disease Using Screening of Multiple Prospective New Treatments. J Parkinsons Dis. 2013 Jan 1;3(3):231–9.
Finding new therapies for Parkinson’s disease (PD) is a slow process. We assembled an international committee of experts to examine drugs potentially suitable for repurposing to modify PD progression. This committee evaluated multiple drugs currently used, or being developed, in other therapeutic areas, as well as considering several natural, non-pharmaceutical compounds. The committee prioritized which of these putative treatments were most suited to move immediately into pilot clinical trials. Aspects considered included known modes of action, safety, blood-brain-barrier penetration, preclinical data in animal models of PD and the possibility to monitor target engagement in the brain. Of the 26 potential interventions, 10 were considered worth moving forward into small, parallel ‘learning’ clinical trials in PD patients. These trials could be funded in a multitude of ways through support from industry, research grants and directed philanthropic donations. The committee-based approach to select the candidate compounds might help rapidly identify new potential PD treatment strategies for use in clinical trials.
Friday 1 December 2017
Correlation between the availability of dopamine transporter and olfactory function in healthy subjects
This is a nice article which showcases how making data available prevents unnecessary replication and expense. The Parkinson's Progression Markers Initiative 'dumped' (in a good way) a large proportion of the data in ~400 PD patients and ~200 controls online for researchers in the field to devour.
Here the authors confirm findings that up to now have only been available in small numbers of participants and they demonstrate the relationship between smell test scores and striatal binding. Age, as it is in so many exposure-outcome associations, was an important confounding factor. However, even after taking account of age in the analysis there was a clear association between sense of smell and binding ratio on DAT-SPECT.
Interesting to me was the fact that age had a stronger relationship with caudate binding compared with putaminal binding, whereas olfaction was equally associated with caudate and putaminal binding. I guess one could raise questions about the legitimacy of using linear analysis methods when the distribution of smell data is clearly non-normal, but a pragmatic approach must be taken in some instances and if they transformed the smell data here they would ceased to have meaning...
Eur Radiol. 2017 Nov 21. doi: 10.1007/s00330-017-5147-7. [Epub ahead of print]
Pak K, Kim K, Lee MJ, Lee JM, Kim BS, Kim SJ, Kim IJ.
https://link.springer.com/article/10.1007%2Fs00330-017-5147-7
OBJECTIVES: Olfactory dysfunction in Parkinson's disease is usually prodromal to other symptoms. In this study, we aimed to explore the association of olfactory function with the availabilities of striatal dopamine transporter (DAT) in healthy subjects.
METHODS: Data used in the preparation of this article were obtained from Parkinson's Progression Markers Initiative database ( www.ppmi-info.org/data ). The study population consisted of healthy controls with screening 123I-FP-CIT single photon emission tomography (SPECT). University of Pennsylvania Smell Identification Test (UPSIT) was assessed to evaluate the olfactory function.
RESULTS: Totally, 181 healthy subjects (117 male, 64 female) with 123I-FP-CIT SPECT data were included in this study. Specific binding ratios (SBRs) of the caudate nucleus (rho = -0.4217, p < 0.0001), putamen (rho = -0.2292, p = 0.0019), and striatum (rho=-0.3425, p < 0.0001) showed a reduction with ageing. SBRs of the caudate nucleus, putamen, and striatum were positively correlated with UPSIT (rho = 0.3716, p < 0.0001; rho = 0.3655, p < 0.0001; rho = 0.3880, p < 0.0001). After controlling for age by partial correlation, SBRs of the caudate nucleus, putamen, and striatum showed an influence on UPSIT (rho = 0.3288, p < 0.0001; rho = 0.3374, p < 0.0001; rho = 0.3511, p < 0.0001).
CONCLUSION: Olfactory function is associated with the availability of striatal DAT independent of age in healthy subjects.
KEY POINTS: • Olfactory dysfunction in Parkinson's disease is prodromal to other symptoms. • The availability of dopamine transporter showed a reduction with ageing. • Olfactory function is associated with the availability of dopamine transporter.
Here the authors confirm findings that up to now have only been available in small numbers of participants and they demonstrate the relationship between smell test scores and striatal binding. Age, as it is in so many exposure-outcome associations, was an important confounding factor. However, even after taking account of age in the analysis there was a clear association between sense of smell and binding ratio on DAT-SPECT.
Interesting to me was the fact that age had a stronger relationship with caudate binding compared with putaminal binding, whereas olfaction was equally associated with caudate and putaminal binding. I guess one could raise questions about the legitimacy of using linear analysis methods when the distribution of smell data is clearly non-normal, but a pragmatic approach must be taken in some instances and if they transformed the smell data here they would ceased to have meaning...
Eur Radiol. 2017 Nov 21. doi: 10.1007/s00330-017-5147-7. [Epub ahead of print]
Pak K, Kim K, Lee MJ, Lee JM, Kim BS, Kim SJ, Kim IJ.
https://link.springer.com/article/10.1007%2Fs00330-017-5147-7
OBJECTIVES: Olfactory dysfunction in Parkinson's disease is usually prodromal to other symptoms. In this study, we aimed to explore the association of olfactory function with the availabilities of striatal dopamine transporter (DAT) in healthy subjects.
METHODS: Data used in the preparation of this article were obtained from Parkinson's Progression Markers Initiative database ( www.ppmi-info.org/data ). The study population consisted of healthy controls with screening 123I-FP-CIT single photon emission tomography (SPECT). University of Pennsylvania Smell Identification Test (UPSIT) was assessed to evaluate the olfactory function.
RESULTS: Totally, 181 healthy subjects (117 male, 64 female) with 123I-FP-CIT SPECT data were included in this study. Specific binding ratios (SBRs) of the caudate nucleus (rho = -0.4217, p < 0.0001), putamen (rho = -0.2292, p = 0.0019), and striatum (rho=-0.3425, p < 0.0001) showed a reduction with ageing. SBRs of the caudate nucleus, putamen, and striatum were positively correlated with UPSIT (rho = 0.3716, p < 0.0001; rho = 0.3655, p < 0.0001; rho = 0.3880, p < 0.0001). After controlling for age by partial correlation, SBRs of the caudate nucleus, putamen, and striatum showed an influence on UPSIT (rho = 0.3288, p < 0.0001; rho = 0.3374, p < 0.0001; rho = 0.3511, p < 0.0001).
CONCLUSION: Olfactory function is associated with the availability of striatal DAT independent of age in healthy subjects.
KEY POINTS: • Olfactory dysfunction in Parkinson's disease is prodromal to other symptoms. • The availability of dopamine transporter showed a reduction with ageing. • Olfactory function is associated with the availability of dopamine transporter.
Wake up and smell the coffee!!
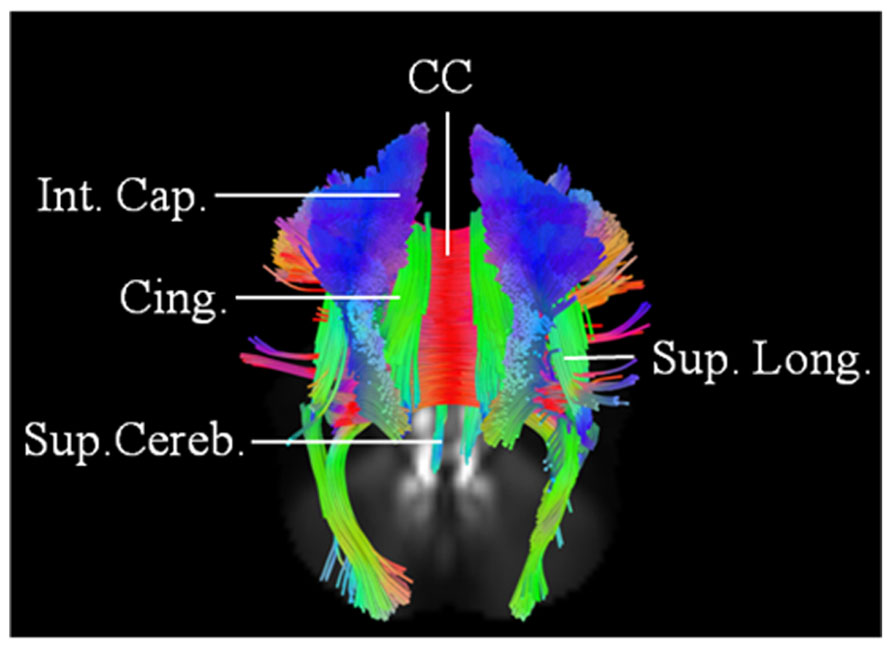
We've been talking about smell loss and the best ways to measure for it at PREDICT-PD recently. Hyposmia or anosmia is the most common non-motor symptom in Parkinsons patients and can be present many years before diagnosis. We can test smell formally with various tests, the most widely used being the University of Pennsylvania smell test (UPSIT), which tests 40 common odours including coffee, chocolate and strawberries.
It is proposed that this symptom indicates that the neurodegenerative process in PD starts outside of the brain itself and perhaps in the peripheral nervous system but there are few studies which directly address the reasons for smell loss and the underlying mechanisms. MRI is a good candidate for this - recent studies have used high resolution MRI to look more closely at problems in the visual systems in Parkinsons and the study below, by researchers in Iran and Switzerland uses MRI to ask whether there are imaging findings associated with smell loss.
They use the large cohort study PPMI, which has recruited patients with early Parkinsons and used a technique called connectometry, which allows analysis of white matter tracts, the main pathways in the brain. A number of pathways were found to be significantly different between patients with different levels of smell loss. Tracts involving the limbic system, which mediates emotion, memory and some aspects of behaviour were particularly sensitive for early smell loss. This is an interesting finding as these areas are not traditionally associated with Parkinsons.
This is early work but as new techniques for MRI analysis continue to be developed and our ability to image these previously unseen parts of the brain develops we are getting a fuller picture of why these symptoms develop, how to measure them and fundamentally, how to prevent their degeneration.
Brain Imaging Behav. 2017 Nov 13. doi: 10.1007/s11682-017-9781-0. [Epub ahead of print]
Exploring white matter microstructure and olfaction dysfunction in early parkinson disease: diffusion MRI reveals new insight.
Sobhani S, Rahmani F, Aarabi MH, Sadr AV.
Wednesday 29 November 2017
Food for thought
The race is on, and waiting for the start gun is a loser’s strategy!
At least this is true in the race to beat neurodegenerative diseases
such as Alzheimer’s disease or Parkinson’s. Many studies to stop or slow these
conditions have failed when they recruit people with established disease. By
this point the disease-causing proteins (β-amyloid and tau in the case of Alzheimer’s
and α-synuclein in the case of Parkinson’s) are too widespread for benefit to
be noticeable. The best metaphor I’ve heard is that it is easier to blow out a
match than put out a forest fire.
This week sees the open access publication of a nutritional supplement
for prodromal Alzheimer’s disease. Participants were recruited if they
fulfilled one of the internationally agreed definitions of Prodromal
Alzheimers. To do this they needed some memory impairment that wasn’t severe
enough to impact on their daily life, and some other evidence of underlying
Alzheimer’s. This could be MRI, amyloid-specific PET scans or spinal fluid
findings supportive of Alzheimer’s. Participants either drank a nutritional
supplement, that has been designed to give large doses of micronutrients that
theoretically counteract some of the damage caused by Alzheimers, or a similar
tasting placebo drink. They had neuropsychological assessments at 6 months, 1
year and 2 years from the start.
Unfortunately, this study didn’t reach its primary endpoint of improved
composite scores of the neuropsychological testing. However, it also had
several secondary outcomes, and they did find that the people taking the
supplement had a relative preservation of the hippocampal volume on MRI scans
after 2 years compared to those taking placebo. The hippocampus is a brain structure
that is central to memory, and is usually found to be shrunken in brain scans
of people with Alzheimer’s.
So is this study the death knell for the supplement, especially after studies
of its effects in people with established disease also failed to meet their
primary target? Well, not necessarily. This study used ‘surrogate outcome
measures’. The real question in disease preventing trials is: did the
intervention prevent the disease? When dealing with a ‘prodromal’ or ‘prediagnostic’
condition, this might take many years. Clinical trials are incredibily
expensive and labour intensive to run and so often use surrogate measures to
shorten the duration of the study. This is akin to saying “I can run a mile in
5 minutes, therefore I can run a marathon in 2 hours 10 minutes.” This is
misleading, as the two are not necessarily compatible enough to know that
performance in one test is indicative of the other. Secondly, the fact that
there was less shrinking of a key, and relevant, brain structure suggests that
there might be some benefit. There are extension studies that are ongoing and
data will be published when those are complete. We eagerly await their results.
As a final thought, this study lends strong support to our endeavours at
PREDICT-PD, and those of others around the world. There is a strong desire to
get a robust method of diagnosing Parkinson’s. This is not just an abstract
academic exercise, but is of key concern to the public and pre-diagnostic
states are being carefully examined by industry too. It is essential to bring
together all three groups of stakeholders if we are to succeed in our fight
against these neurodegenerative diseases.
RNR
Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K,
Kivipelto M, et al. 24-month intervention with a specific multinutrient in
people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind,
controlled trial. The Lancet Neurology. 2017 Dec;16(12):965–75.
BACKGROUND:Nutrition is an important modifiable risk factor
in Alzheimer's disease. Previous trials of the multinutrient Fortasyn Connect
showed benefits in mild Alzheimer's disease dementia. LipiDiDiet investigated
the effects of Fortasyn Connect on cognition and related measures in prodromal
Alzheimer's disease. Here, we report the 24-month results of the trial.
METHODS:LipiDiDiet was a 24-month randomised, controlled,
double-blind, parallel-group, multicentre trial (11 sites in Finland, Germany,
the Netherlands, and Sweden), with optional 12-month double-blind extensions.
The trial enrolled individuals with prodromal Alzheimer's disease, defined
according to the International Working Group (IWG)-1 criteria. Participants
were randomly assigned (1:1) to active product (125 mL once-a-day drink
containing Fortasyn Connect) or control product. Randomisation was
computer-generated centrally in blocks of four, stratified by site. All study
personnel and participants were masked to treatment assignment. The primary
endpoint was change in a neuropsychological test battery (NTB) score. Analysis
was by modified intention to treat. Safety analyses included all participants
who consumed at least one study product dose. This trial is registered with the
Dutch Trial Register, number NTR1705.
FINDINGS:Between April 20, 2009, and July 3, 2013, 311 of
382 participants screened were randomly assigned to the active group (n=153) or
control group (n=158). Mean change in NTB primary endpoint was -0·028 (SD
0·453) in the active group and -0·108 (0·528) in the control group; estimated
mean treatment difference was 0·098 (95% CI -0·041 to 0·237; p=0·166). The
decline in the control group was less than the prestudy estimate of -0·4 during
24 months. 66 (21%) participants dropped out of the study. Serious adverse
events occurred in 34 (22%) participants in the active group and 30 (19%) in
control group (p=0·487), none of which were regarded as related to the study
intervention.
INTERPRETATION:The intervention had no significant effect on
the NTB primary endpoint over 2 years in prodromal Alzheimer's disease.
However, cognitive decline in this population was much lower than expected,
rendering the primary endpoint inadequately powered. Group differences on
secondary endpoints of disease progression measuring cognition and function and
hippocampal atrophy were observed. Further study of nutritional approaches with
larger sample sizes, longer duration, or a primary endpoint more sensitive in
this pre-dementia population, is needed.
FUNDING:European Commission 7th Framework Programme.
Subscribe to:
Posts (Atom)
Mild Parkinsonian Signs in a Community Population
One question that many of the PREDICT-PD participants ask me is “I am slower than I used to be, does it mean that I am getting Parkinson’...
-
Motor and non-motor correlates of olfactory dysfunction in Parkinson's disease. Berendse HW , Roos DS , Raijmakers P , Doty RL . J...
-
What motivates Parkinson's disease patients to enter clinical trials? Valadas A, Coelho M, Mestre T et al. Parkinsonism Relat Disord....